Oxygen 16 Protons Neutrons Electrons
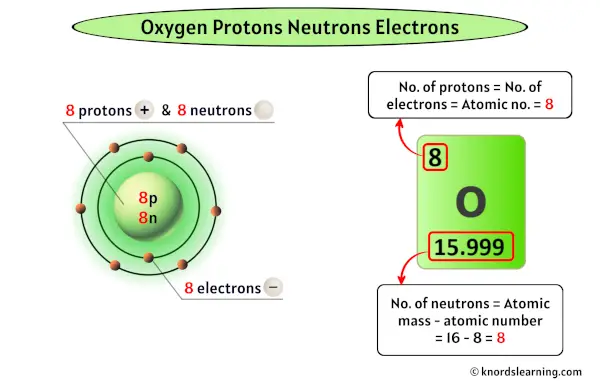
Oxygen has eight protons, viii neutrons and viii electrons.
But how volition yous observe the number of protons, neutrons and electrons in Oxygen (O)?
Well, information technology is very piece of cake to find the protons, neutrons and electrons of oxygen atom.
Here I take given a very simple method for finding the protons, neutrons and electrons of oxygen atom.
Let's dive right into it!
Finding the Protons, Neutrons and Electrons in Oxygen
How to find protons?
- The number of protons can be establish by knowing the atomic number of that atom.
How to find neutrons?
- The number of neutrons can exist found by subtracting the diminutive number from its atomic mass.
How to find electrons?
- For a neutral atom, the number of electrons tin exist found by knowing the diminutive number of that atom.
Permit's calculate the number of protons, neutrons and electrons in oxygen.
#1 Number of Protons in Oxygen
If y'all accept a periodic table with yous, then almost of the answers are in forepart of you.

You can run across the elements, their diminutive number and their atomic mass from the periodic table.
Now here our chemical element is Oxygen (O).
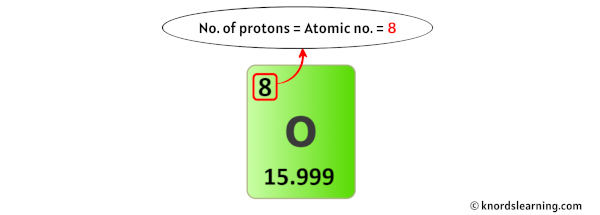
So from the to a higher place periodic tabular array, yous can see that the atomic number of oxygen is 8.
As the atomic number of oxygen is 8, information technology has a total of viii protons in its nucleus.
Thus, the number of protons in Oxygen is viii.
#two Number of Neutrons in Oxygen
In social club to find the number of neutrons of an oxygen cantlet, you should know its diminutive mass first.
The number of neutrons in oxygen tin be obtained by subtracting the diminutive number from its diminutive mass.
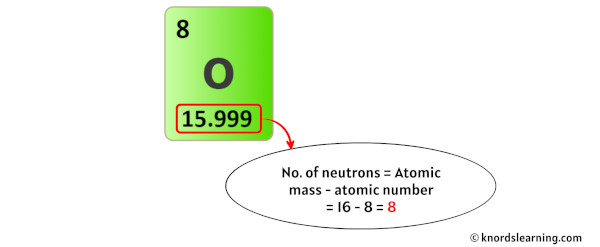
The atomic mass of oxygen is 15.999 u (which you can round it to 16).
And so from this atomic mass (i.due east 16), you accept to subtract its atomic number (i.eastward 8).
So you volition become xvi – 8 = 8.
Thus, the number of neutrons in Oxygen is viii.
#3 Number of Electrons in Oxygen
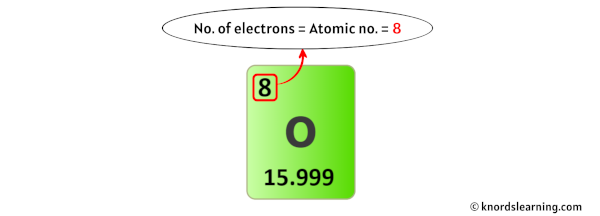
For a neutral atom, the number of electrons and the number of protons are the aforementioned.
Hither, the oxygen atom is a neutral atom.
And then the number of electrons in oxygen is equal to its number of protons (which is as well equal to its atomic number).
In the beginning, we have found that the number of protons in oxygen is eight.
Thus, the number of electrons in Oxygen is 8.
Summary
Number of Protons in Oxygen
- The number of protons can be found by knowing the atomic number of that atom.
- Number of Protons in Oxygen = Atomic number of Oxygen = eight
Number of Neutrons in Oxygen
- The number of neutrons can be found by subtracting the diminutive number from its atomic mass.
- Number of Neutrons in Oxygen = Diminutive mass of Oxygen – Atomic number of Oxygen = 16 – 8 = 8
Number of Electrons in Oxygen
- For a neutral atom, the number of electrons can exist found by knowing the atomic number of that cantlet.
- Number of Electrons in Oxygen = Atomic number of Oxygen = eight
I hope yous accept understood the simple method for finding the protons, neutrons and electrons of oxygen atom.
Check out related topics for more practice;
Fluorine protons neutrons electrons
Neon protons neutrons electrons
Aluminum protons neutrons electrons
Silicon protons neutrons electrons
Phosphorus protons neutrons electrons
Oxygen 16 Protons Neutrons Electrons,
Source: https://knordslearning.com/oxygen-protons-neutrons-electrons/
Posted by: kingrepasustem.blogspot.com


0 Response to "Oxygen 16 Protons Neutrons Electrons"
Post a Comment